Strategies for Digitalisation in Pathology: Cogito Ergo I Digitise
DOI: https://doi.org/10.47184/tp.2025.01.01This article examines the transformative impact of digitalisation in pathology by exploring the challenges of integrating digital processes within laboratories. It emphasises the importance of detailed planning, structured data capture, and interdisciplinary collaboration – including the strategic use of artificial intelligence – to navigate and overcome complex barriers.
Keywords: documentation, AI, workflow, change management, structural changes
Today, there is no one who has not already heard the term “digitalisation”. However, what is understood by it varies more than ever. While some rejoice that they can meet their loved ones face-to-face via video call in even the most remote locations around the world, Chinese researchers, as early as 2021, demonstrated that a person’s cardiovascular risk can be more accurately assessed by using Artificial Intelligence (AI) to analyse facial images than by relying on the current medical gold standards [1]. The revolution is not driven by the mere availability of technology, but by the magnitude of benefits achieved, when new technology is concretely implemented in specific application areas.
In general, there is the impression that many pathology labs have taken only minimal steps toward digitalisation over the past decades, and standard processes have not changed significantly. The number of diagnostic biomarkers in immunohistochemistry and molecular pathology grows continuously and extensive research has increased the complexity of diagnostics to the patients benefit
Documentation
The greatest challenge for laboratory personnel, physicians, and staff remains the consolidated documentation of all information and making it accessible to everyone. These following examples illustrate how barriers in information capture and communication can lead to issues:
Example 1
If the billing staff cannot determine how many and what types of materials were used or produced, significant time is spent obtaining that information. In the worst case, items might not be recorded at all, causing the case value to drop. If the pathologist dictates the numbers personally, valuable working time is lost in translating standardised information into standardised values.
Example 2
If laboratory staff handwrite the numbering on cassettes and slides, potential error sources that affect patient safety emerge. Human errors are inevitable with repetitive tasks – regardless of intelligence or capability, though those factors may affect the error frequency. And even if the number is legibly written, is that a 3 or a 9? Not to mention that handwritten documentation prevents the clear presentation and filtering of data (e. g. determining who last processed the third block of the fourth slide of case 43286).
Example 3
For pathologists – as with any physician – the common is common and the rare is rare. Automatic measurement of tumor thickness and distances can now be performed just as seamlessly as algorithm-based cell counting in immunohistochemistry, without delay. It leaves more time for the intriguing, rare cases that require a more detailed evaluation or even additional research or discussion.
The Role of AI
All three examples illustrate some of the many areas in the pathology lab where digitalisation plays a role. The final point alludes to one of the most recent revolutions: AI. Although not everyone has yet had even an initial encounter with Large Language Models (LLMs) such as ChatGPT, Gemini or Claude, LLMs today pass the Turing Test that was developed around 1950 [2, 3]. According to Turing’s test, machines achieve human-like intelligence when, in a blind conversation, the other participant can no longer distinguish whether they are communicating with a human or a machine.
In digitalisation of pathology, the focus is not primarily on the use of AI, as is often claimed. Rather, AI only becomes relevant at the end of the digitalisation process – and only under two conditions. First, before AI integration, all relevant data must be captured digitally and in a structured manner, because AI can only operate on digital data. Second, the entire AI development process (including use case, architecture, and training) and the subsequent integration of AI are crucial to delivering added value. If the AI’s task is not well defined, or if the AI performs it poorly, it is as useless as an AI model that is not sensibly integrated into the user’s workflow.
Workflow Digitalisation
When defining the process pathway, it is essential to consider the complete workflow – from sample collection to archiving. If information is not captured digitally at its point of creation, it must later be converted. If digitalisation is missed at one stage, the overall result can be considerably compromised. With approximately 200 to 300 work steps from sample collection to case closure, a complex baseline situation arises. Workflows will subsequently change significantly as new steps are added and existing ones are eliminated. Established methods should be initially employed to document current workflows, and these same methods should be utilised to conceptualise and document future workflows.
In pathology, there are a total of 24 product categories that play a role in digitalisation. One of the most important is the slide scanner. According to our research, there are over 80 different models from 17 manufacturers on the market. For this single product, the decision-maker must acquire an overview of the market and a clear understanding of the evaluation criteria to make an informed decision. Simple criteria may include dimensions or the availability of breakdown services. Other obvious factors are price, scanning speed, or scan mode. Less apparent, however, is the detailed knowledge of individual products and differences in scanner handling, which may have long-term impacts on both costs and workflow. Informed decision making is particularly challenging given the number of product categories, many market players, and differences between the products.
Once well-informed decisions have been made, the procured hardware and software must be integrated. As many process steps change or disappear, a realignment of overall positioning is often advisable to reduce walking distances (Fig. 1).
An appropriate method should be used to first capture the current positioning and then to visualise the new configuration. Since digitalisation often involves many participants, initial planning and documentation of processes and the spatial layout is a major support for ensuring all stakeholders understand and communicate effectively.
Change Management
Digitalisation not only demands significant know-how but also the early involvement of all stakeholders to address fears and ensure collective buy-in. Research shows that 70 % of all organisational transformations fail or fall short of expectations due to insufficient attention to change management [4]. To address this, the five-phase model developed by Wilfried Krüger (2014) offers a practical framework for managing change and provides a roadmap for navigating complex transformations. Change management in pathology digitalisation is a structured process that begins with identifying the need for transformation and understanding potential barriers within an organisation. Early and transparent communication is essential to build support – painting a clear picture of how digitalisation can reduce repetitive tasks [5]. Consequently, a professional project management framework should be established right from the start.
Beyond changes in individual work steps, there are cross-sectional topics. The largest of these is IT infrastructure. An adequate server and data-transfer infrastructure must be in place to manage the load from large data volumes of typically one to three gigabytes per slide. Other cross-sectional matters include the laboratory information system, quality management, and more.
Structural Change
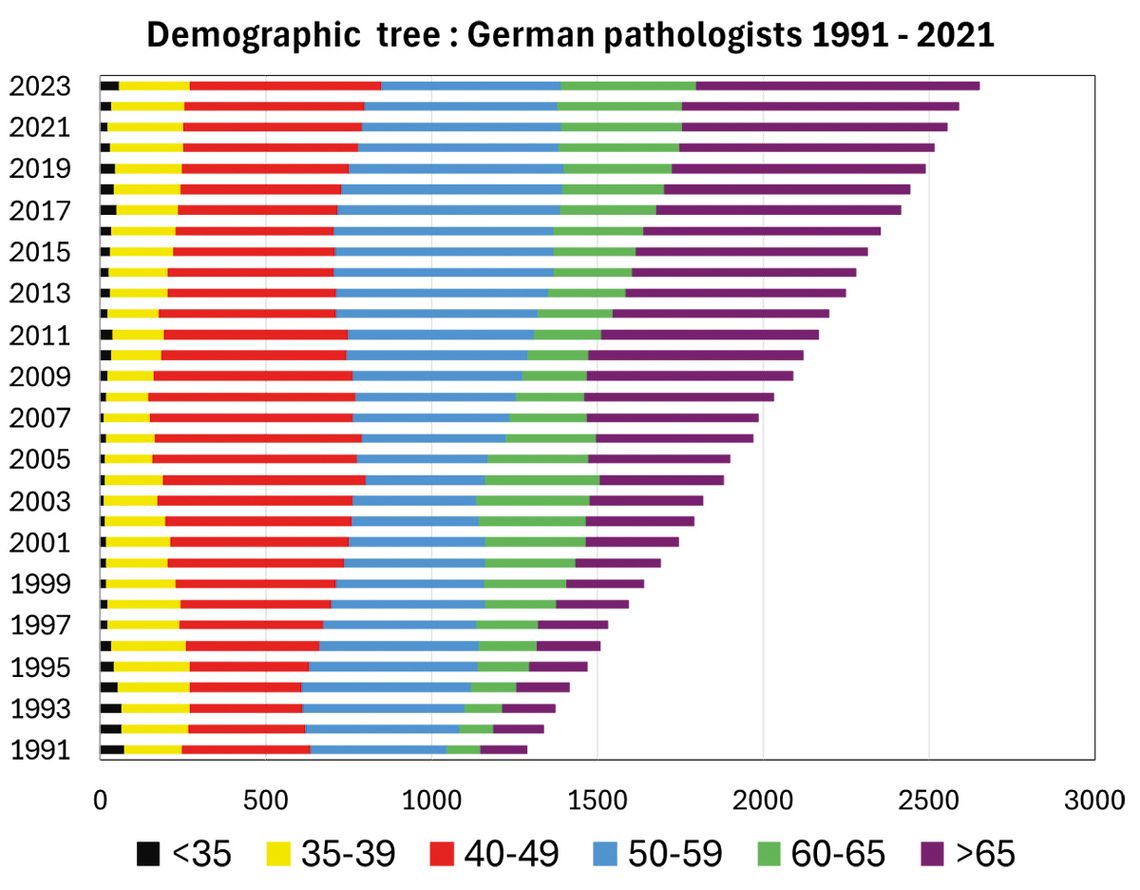
For pathology, digitalisation represents a complex structural change that requires substantial effort to implement. This is further complicated by external circumstances. A 2021 study at German university clinics, for example, revealed an average increase in cases of 26 % over the past ten years [6]. This growth is consistent with data from the UK, which indicates an annual increase of 4.5 % since 2007 – a considerably higher increase [7]. In a study, 17 out of 18 university institutes in Germany responding to the questionnaire reported that they were unable to adequately manage the case volumes [6]. At the same time, there is a demographic peculiarity: pathologists in Germany are getting older, while the number of younger pathologists remains constant (Fig. 2).
Besides the impact of high workloads on diagnostic quality, there is evidence of the already heavy burden on pathologists from routine work. More-over, many are decision makers in the digitalisation process. In the interplay between the complex baseline challenges of digitalisation and the heavy workload faced by those primarily responsible, practical and efficient solution strategies are needed to support the structural transformation.