Roche Diagnostics is a global leader in providing diagnostics, instruments, and reagents. Our
portfolio offers a wide range of solutions for research, laboratory automation, digital diagnostics,
decision support and consulting.
Our vision for Next Generation Sequencing (NGS) is to standardize and simplify sequencing. We
want to equip physicians and scientists with tools to investigate the underlying mechanisms of
various diseases, including rare hereditary diseases, cancers, neonatal disorders, and infectious
diseases, at a molecular level. With our NGS product portfolio and the service from Foundation
Medicine, we support this approach and offer the right solution for your research or diagnostic
testing.
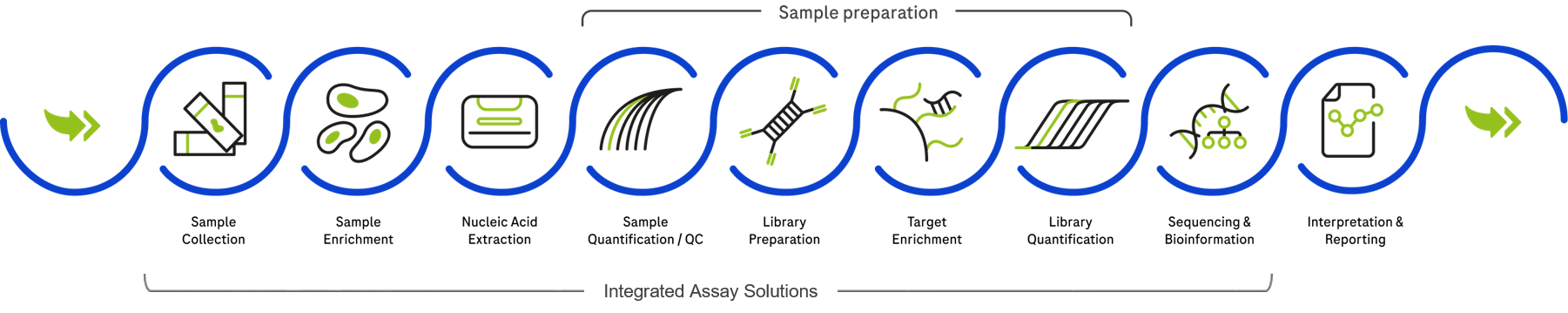
The Roche portfolio comprises a wide array of systems, reagents, digital solutions, and services for the sequencing workflow. Take advantage of our efficient solutions to advance your laboratory and get to know our broad portfolio in our Sequencing Store. Use our Roche Solution Navigator to learn more about our solutions and the possibility of automation.

68305 Mannheim / Germany
Highlights of our Sequencing Product Portfolio:
Comprehensive Genomic Profiling of Tumors with the Services of Foundation Medicine:
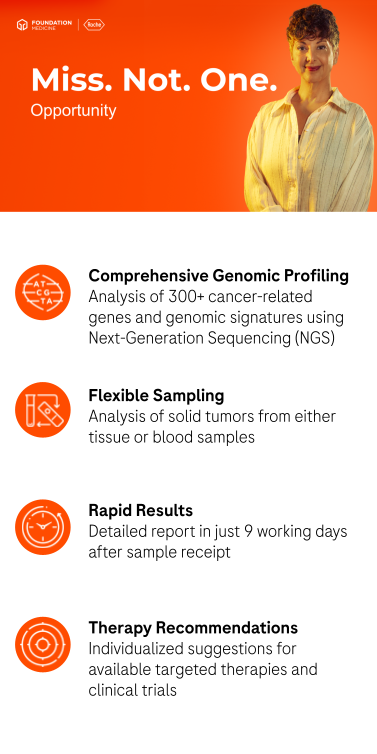
Foundation Medicine specializes in comprehensive genomic profiling of tumors to support personalized therapeutic decisions. Based on a tissue or blood sample, our genetic tests provide valuable insights into the molecular characteristics of solid tumors.
The collection of a blood sample represents a minimally invasive alternative to a tissue biopsy. It enables the analysis of circulating tumor DNA (ctDNA), which allows for testing even when a tissue sample cannot be obtained. Furthermore, in some cases, a liquid biopsy containing ctDNA can provide a more comprehensive picture of tumor heterogeneity than a single tissue sample.
Submit your patient’s sample and receive a well-structured, detailed report with a customized summary of relevant therapies and clinical trials—in just 9 working days after sample receipt.
Our Test Portfolio:
FoundationOne®CDx
Based on an FFPE tissue sample, this test identifies existing genomic alterations (base substitutions, insertions and deletions, copy number variations, and gene arrangements) in over 300 clinically relevant genes, as well as important genomic signatures, including tumor mutational burden (TMB), microsatellite instability (MSI), loss of heterozygosity (LOH), and homologous recombination deficiency (HRD).
FoundationOne®Liquid CDx
Based on a blood sample, this test identifies existing genomic alterations in over 300 clinically relevant genes as well as important genomic signatures, including blood tumor mutational burden (bTMB) and microsatellite instability (MSI). Additionally, the tumor fraction is specified, which provides information on the amount of ctDNA in the blood sample.
Would you like more information? Then visit our Foundation Medicine Website to learn more.
Sequencing by Expansion (SBX): Roche's new sequencing technology for your scientific breakthroughs
SBX combines two forward-looking technologies, unlocking new possibilities in sequencing. During the initial sample preparation step, native DNA is transcribed into an Xpandomer strand. This strand is 50 times longer than the native DNA but preserves its encoded information. The actual sequencing step then takes place: it is Nanopore-based on a High Throughput Sensor Array. The combination of these technologies enables ultra-fast sequencing with high accuracy. It also provides unprecedented flexibility in terms of sample throughput and read length.
Have we sparked your interest? Then visit our SBX Website and learn more about the SBX technology and potential applications.
For more information, please visit:
https://rochesequencingstore.com/
https://rochesequencingstore.com/sequencing-solution-navigator/#/
https://www.roche.de/diagnostik/produkte-loesungen/portfolios/foundation-medicine
https://sequencing.roche.com/global/en/article-listing/sequencing-platform-technologies.html
Download of the brochures by clicking on the picture.





